empowering Qare. anywhere. always.
revolutionizing healthcare with connected care — delivering real-time insights, empowering caregivers, and transforming patient outcomes.
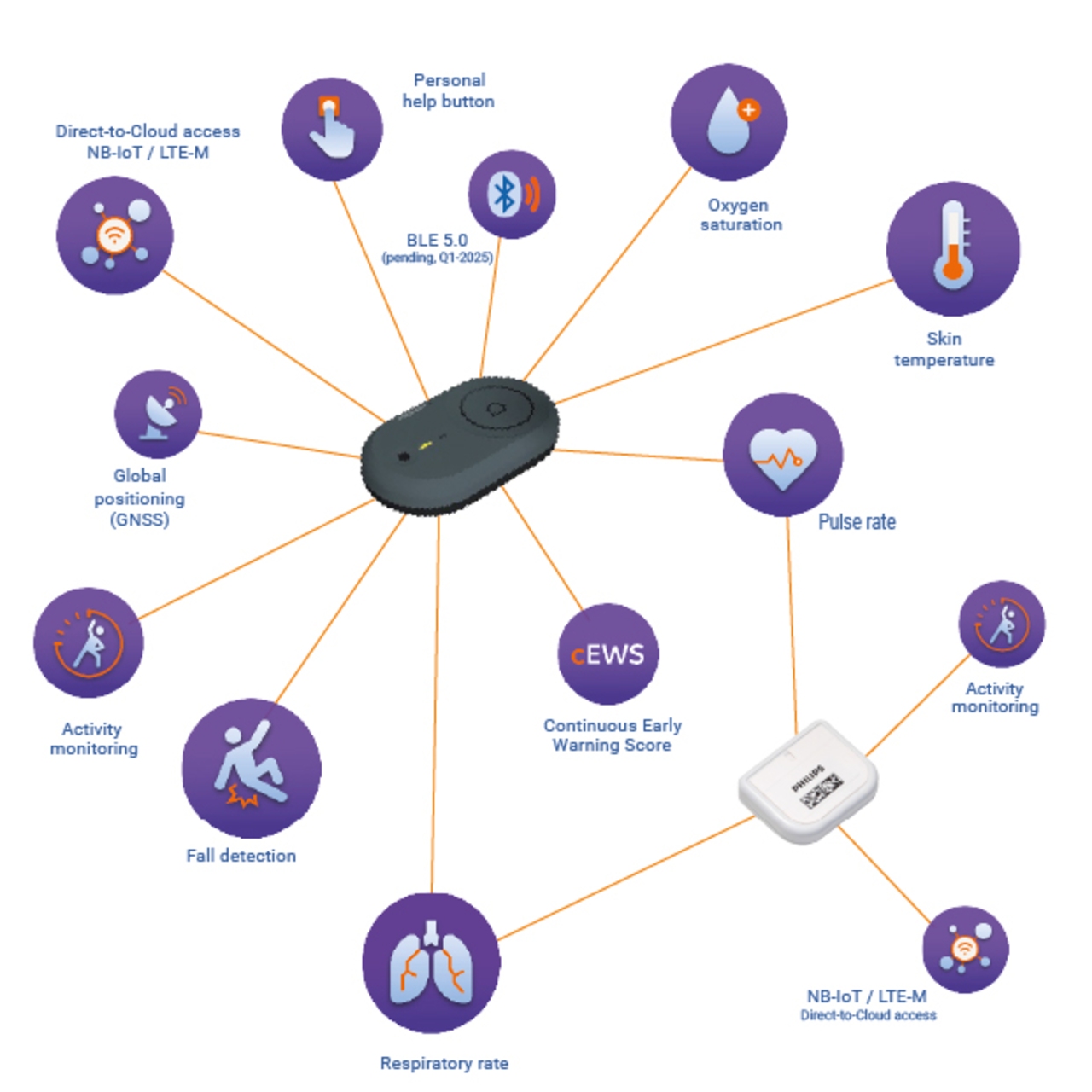
smarter patient monitoring. simplified.
smartQare eliminates the need for spot-check measurements, multiple devices, and complex installations. Our seamless, real-time monitoring solutions enhance efficiency, streamline workflows, and enable timely interventions — giving caregivers the insights they need to act with confidence.
Strategic Partnership Announcement
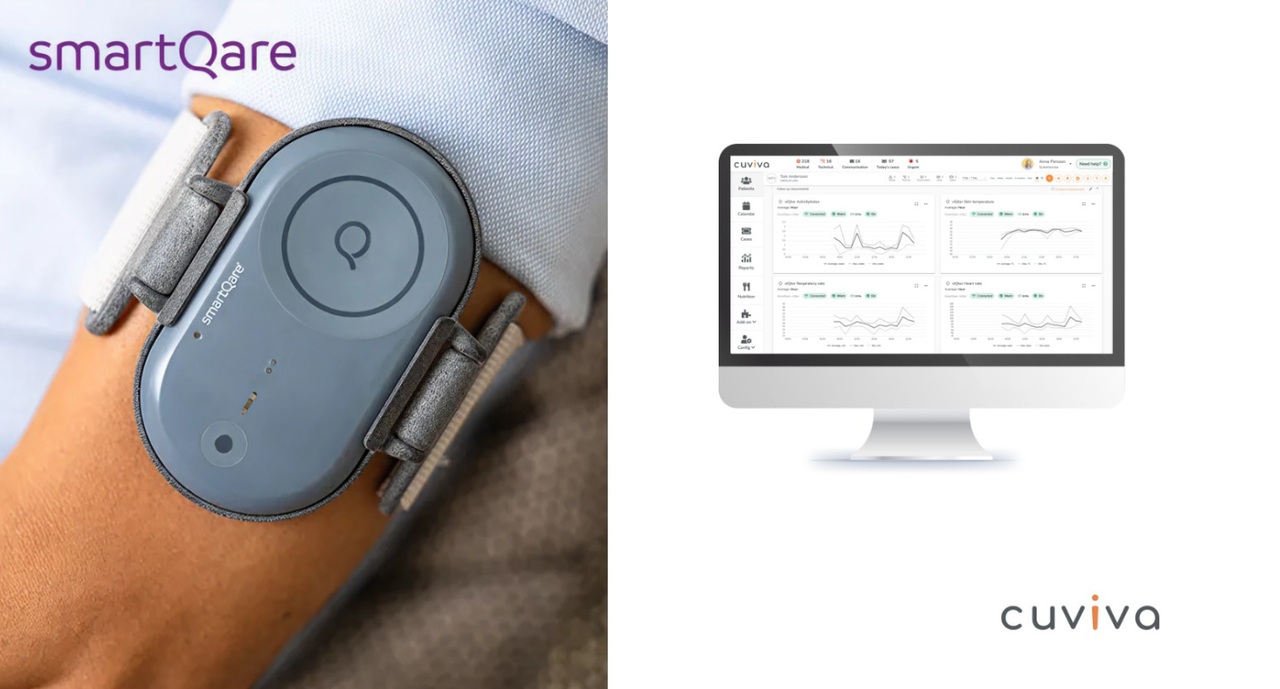
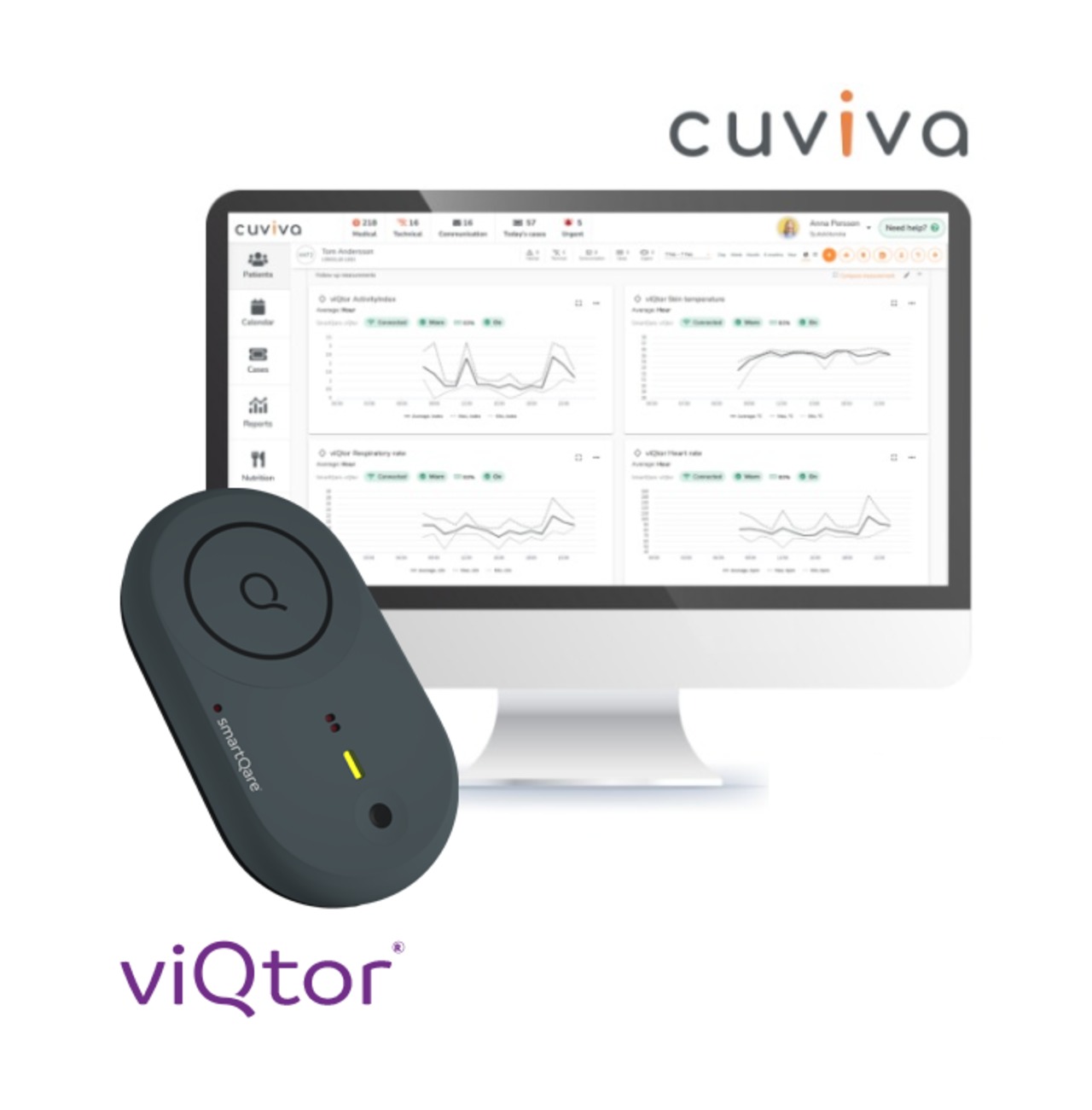
In response to growing pressure on healthcare systems worldwide, Cuviva and smartQare today announced a strategic partnership aimed at transforming how patients are monitored and cared for at home, particularly in the critical period following hospital discharge.
milestone reached!
celebrating 5000 patients monitored with healthdot 3.1
We’re thrilled to announce that today we’ve monitored 5000 patients with Healthdot 3.1 in the Netherlands.

the future of Qare
By unlocking clinical data and harnessing predictive analytics, smartQare empowers healthcare professionals and caregivers with real-time insights — boosting productivity, enabling early interventions, and optimizing healthcare resources across all care settings. The future of care.

PRESSURE – Advancing Remote Cardiovascular Care Beyond the Hospital
PRESSURE (Personal HypErtenSion and Cardiovascular CaRE) is a collaborative innovation project focused on advancing remote patient monitoring for cardiovascular care. This joint initiative brings together smartQare, FreeSenseSolutions, TU/e (EAISI), Catharina Hospital, and Eurocept Homecare, with support from OPZuid, the European Union, and the Province of North Brabant.